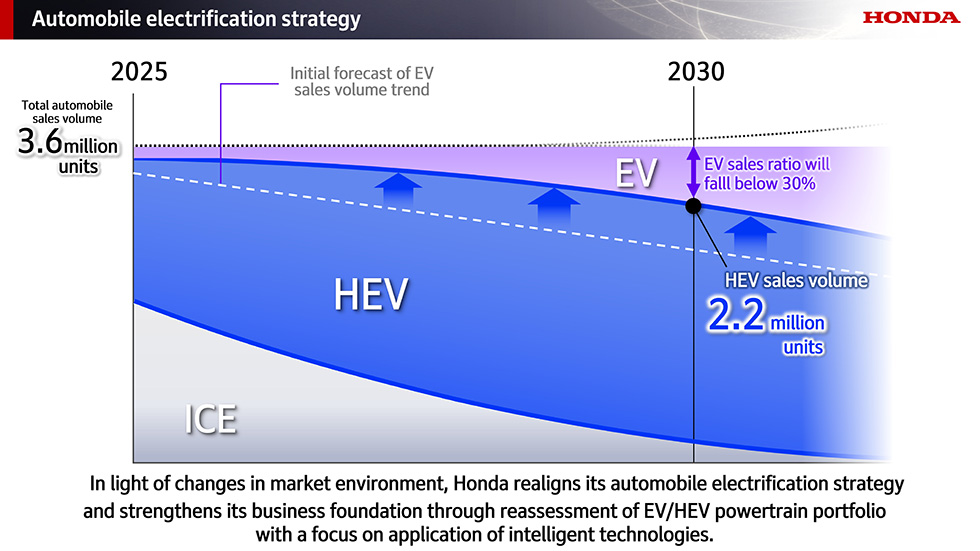
Roche's Columvi expansion bid in danger as FDA's Martin Makary, Richard Pazdur push for more US enrollment in cancer trials
Roche looks unlikely to be able to move its DLBCL drug Columvi earlier in the treatment sequence after experts on an FDA advisory committee joined the agency in questioning the regional imbalance of clinical trial data. What's more, both FDA Commissioner Martin Makary, M.D., and longtime FDA oncology chief Richard Pazdur, M.D., have a new message for drug developers.