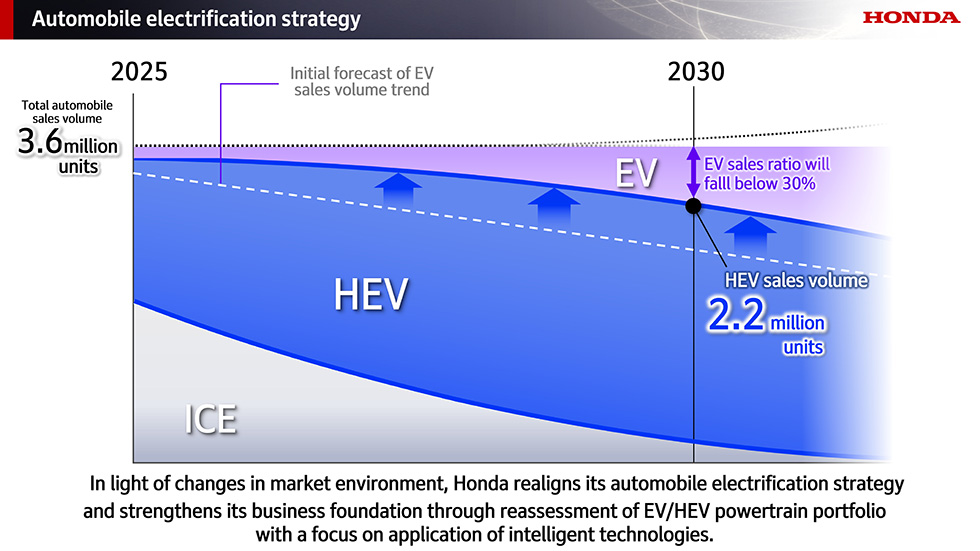
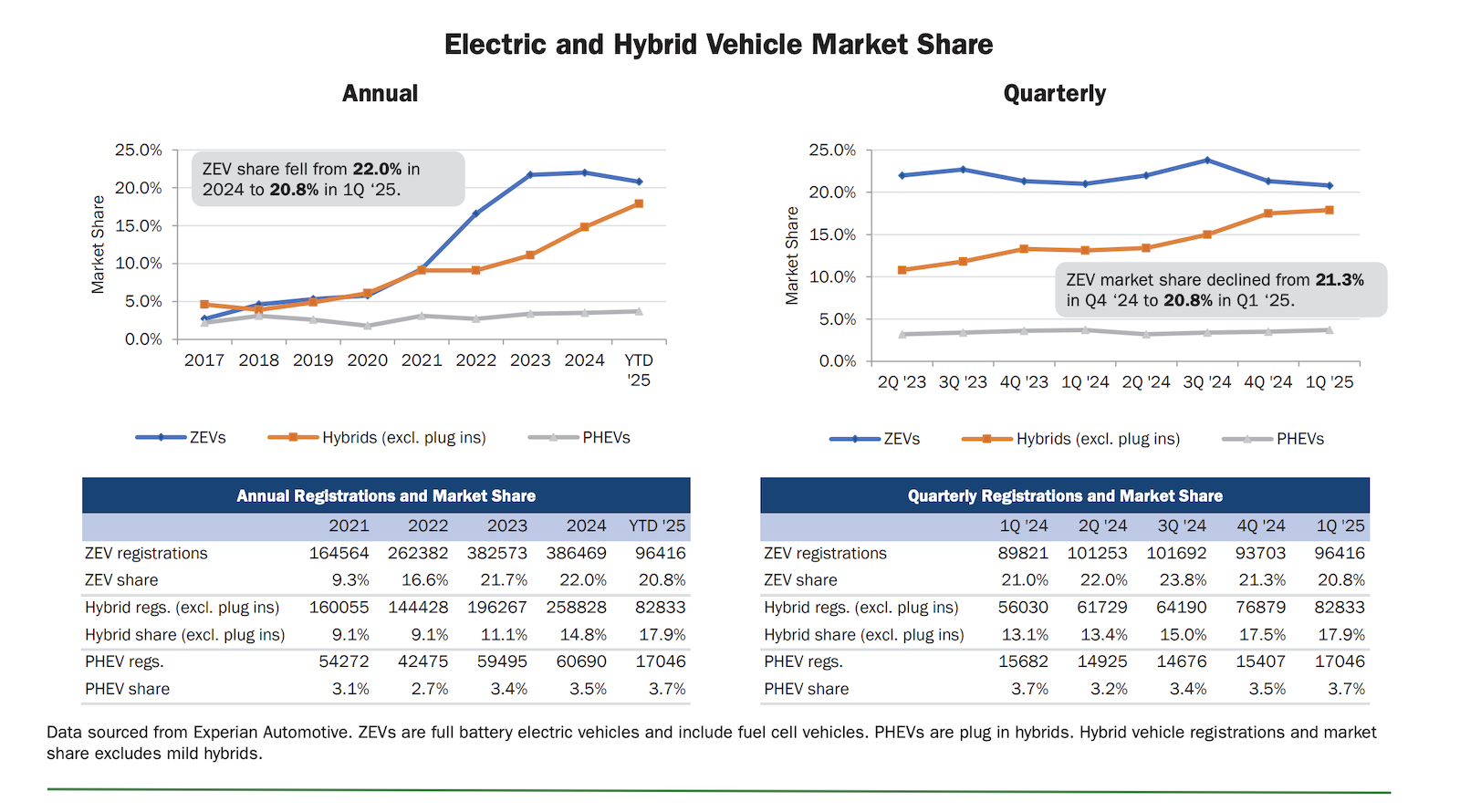
Shorla Oncology Announces FDA Approval of Tepylute 100mg, First and Only Ready-to-Dilute Multi-Dose Vial of Thiotepa
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Shorla Oncology (‘Shorla’), a U.S.-Ireland specialty pharmaceutical company, announced today that the U.S. Food and Drug Administration has granted approval for 100 mg/10mL multi-dose vial of Tepylute...