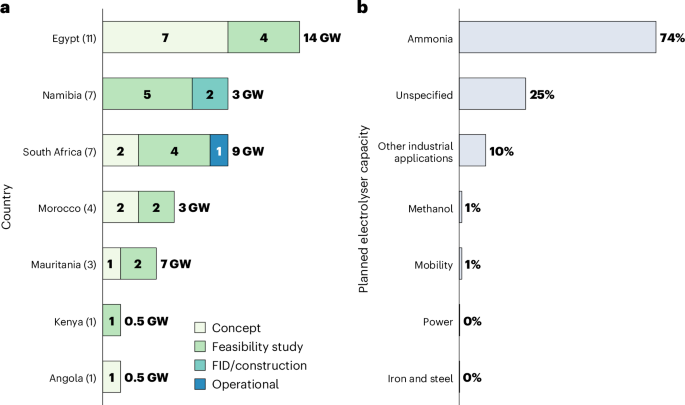
FDA grants breakthrough device status to BiVACOR’s artificial heart
The US Food and Drug Administration (FDA) has granted breakthrough device designation to BiVACOR’s titanium Total Artificial Heart (TAH), as a potential bridge to transplant for the adult population with severe heart failure, where current treatments are insufficient. The post FDA grants breakthrough device status to BiVACOR’s artificial heart appeared first on Medical Device Network.

The post FDA grants breakthrough device status to BiVACOR’s artificial heart appeared first on Medical Device Network.




























![New York City Officially Has Mechanical Garbage Trucks Now [Update]](https://www.jalopnik.com/img/gallery/new-york-city-officially-has-mechanical-garbage-trucks-now/l-intro-1749064637.jpg?#)