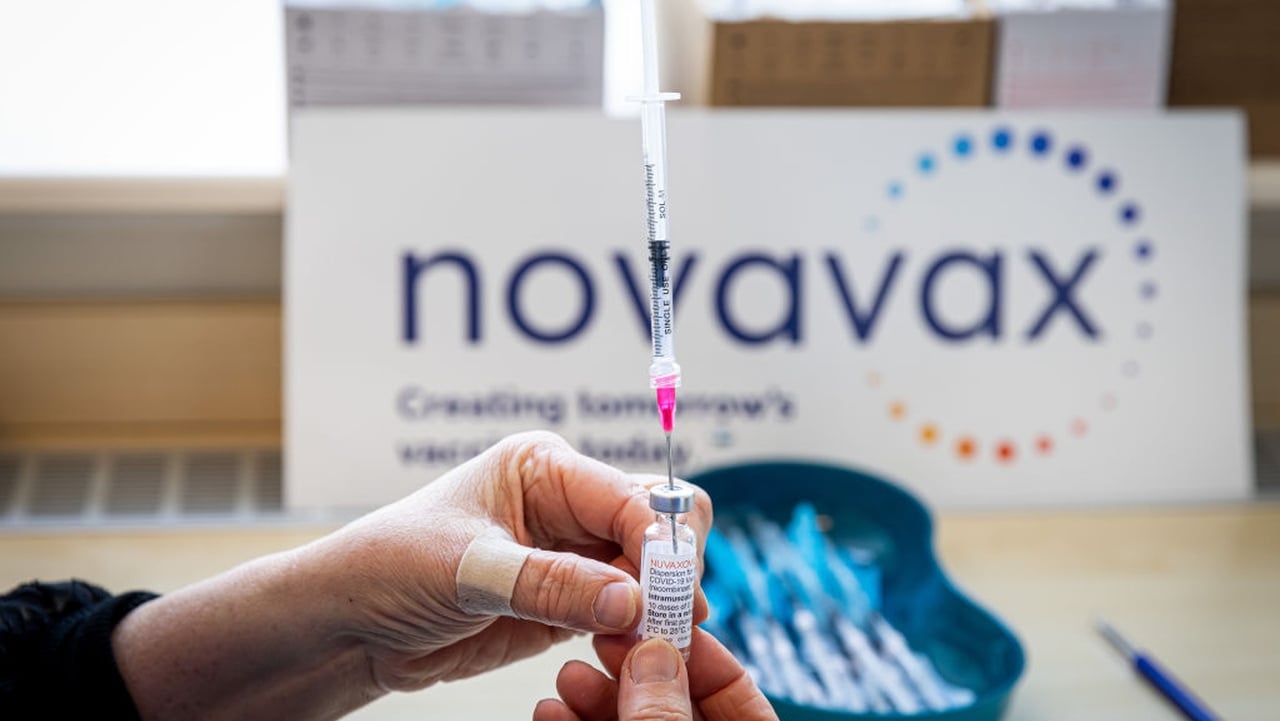
FDA asks Novavax for post-marketing data pledge amid stalled COVID shot review
Despite the FDA missing its deadline to decide on approval for Novavax’s COVID-19 vaccine earlier this month, the company remains confident that its protein-based shot is worthy of a traditional green light.


































































![[Podcast] Behind the Breakthroughs: How Almac Powers Clinical Trial Success with Care](https://imgproxy.divecdn.com/5lAJkli_KcGt1FSsw4EaegjgP76IHREqYEWbhNBJOXw/g:ce/rs:fit:770:435/Z3M6Ly9kaXZlc2l0ZS1zdG9yYWdlL2RpdmVpbWFnZS9CaW9QaGFybWFEaXZlXzEzNDZfeF83MjlfQXJ0d29yay5qcGc=.webp)






























































































.jpg)







