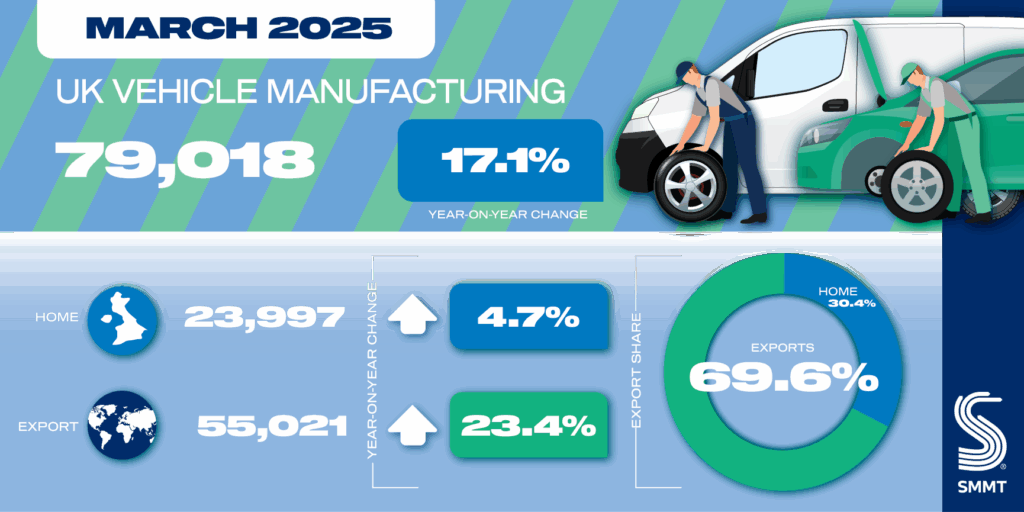
Eisai, Biogen's Leqembi emerges from safety reappraisal unscathed as Europe's CHMP endorses slate of new drugs and label expansions
Europe's Committee for Medicinal Products for Human Use recommended four new drugs for approval, teed up another 16 potential label expansions and—in a major win for Eisai and Biogen—reaffirmed the positive opinion on the Alzheimer’s disease drug Leqembi it first issued in November.