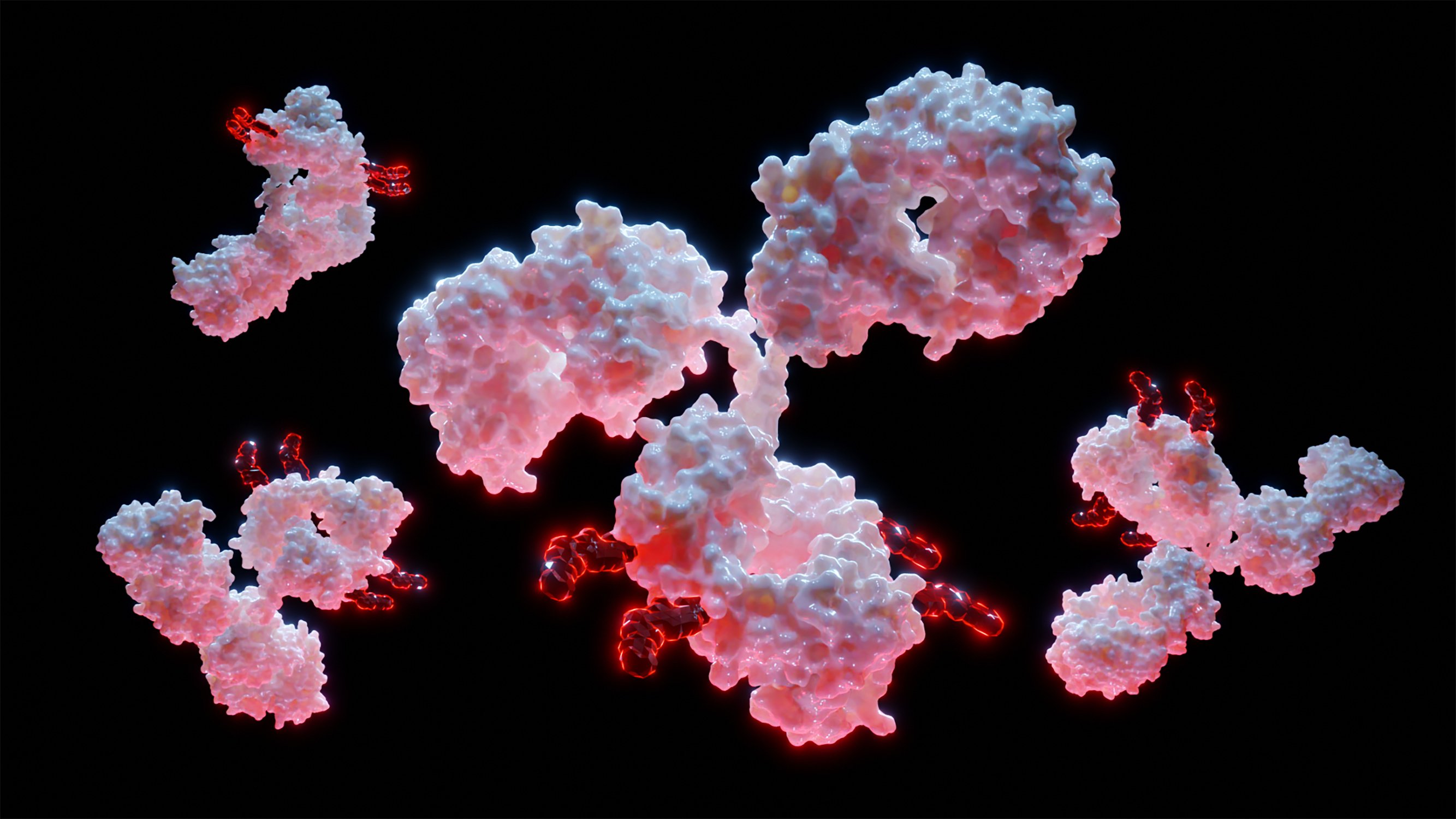
Dual‐Functional Hafnium Oxide Nanoplatform Combining High‐Z Radiosensitization With Bcl‐2 Gene Silencing for Enhanced Cancer Radiotherapy
Advanced Healthcare Materials, Volume 14, Issue 15, 10 June, 2025.

A dual-functional hafnium oxide nanoplatform is developed for enhanced cancer radiotherapy. This innovative system combines the inherent radiosensitizing properties of high-Z hafnium oxide with Bcl-2 gene-silencing capabilities. The nanoplatform demonstrated synergistic enhancement of radiotherapy efficacy through increased generation of reactive oxygen species and suppression of radioresistance mechanisms, achieving significant tumor growth inhibition in a cancer model.
Abstract
The efficacy of radiotherapy is often limited by insufficient radiosensitization and tumor radioresistance. This study reports a dual-functional hafnium oxide nanoplatform that combines high-Z radiosensitization with Bcl-2 gene silencing for enhanced cancer radiotherapy. The nanoplatform is developed by surface modification of hafnium oxide nanoparticles with polyethyleneimine, enabling efficient siRNA delivery while maintaining inherent high-Z radiosensitizing properties. Comprehensive physicochemical characterization confirmed the successful surface modification and stable siRNA complexation. Upon radiation exposure, the nanoplatform enhanced reactive oxygen species generation and DNA damage while simultaneously delivering Bcl-2 siRNA to suppress radioresistance mechanisms. In vitro studies revealed significant enhancement of radiation-induced cell death through synergistic effects of high-Z radiosensitization and Bcl-2 silencing, evidenced by increased γ-H2AX expression and apoptotic cell population. In a murine colon cancer model, the nanoplatform achieved remarkable tumor growth inhibition (80%) when combined with radiotherapy while exhibiting favorable biocompatibility in major organs. Mechanistic studies confirmed effective Bcl-2 downregulation and enhanced DNA damage in tumor tissues, validating this dual-functional therapeutic approach. This study presents a promising strategy for improving radiotherapy outcomes through the simultaneous enhancement of radiosensitization and suppression of radioresistance, potentially advancing the field of cancer radiotherapy.


















































































![Israel drama and good news for the F-35: Paris Air Show Day 1 [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2025/06/IMG_1805-scaled-e1750092662883.jpg?#)
![[Updated] Sudden Deployment of Dozens of U.S. Air Force Tankers Raises Questions](https://theaviationist.com/wp-content/uploads/2025/03/Stratotanker100Years_2-e1750080240327.jpg)