3D Bioprinting and Artificial Intelligence‐Assisted Biofabrication of Personalized Oral Soft Tissue Constructs
Advanced Healthcare Materials, Volume 14, Issue 13, May 16, 2025.

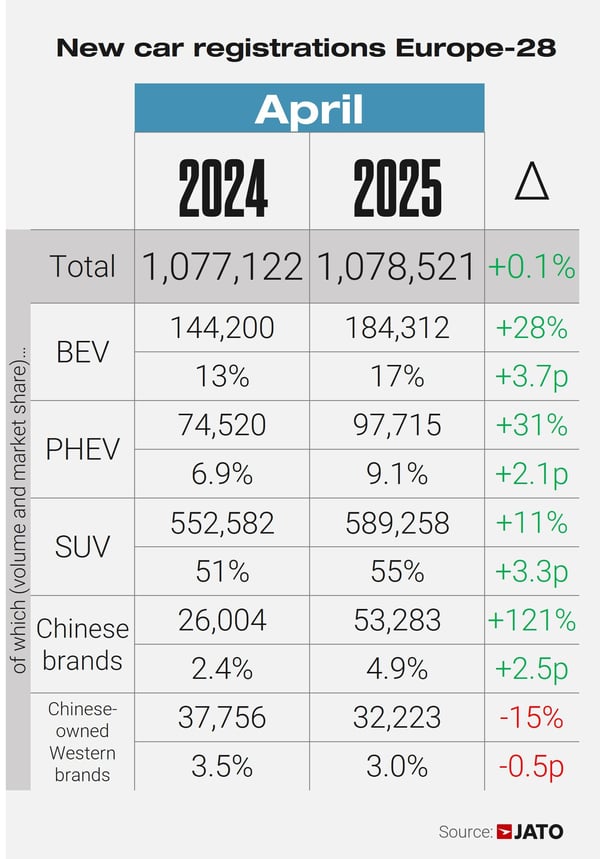
Researchers present a novel 3D bioprinting approach using artificial intelligence (AI) to optimize bioink parameters for fabricating personalized gingival soft tissue constructs. This approach employs polysaccharide/fibrinogen-based bioinks to develop customized, defect-specific grafts, aimed at repairing mucogingival defects from periodontal disease and dental implants. This work also highlights the broader applications of bioprinting and AI in personalized soft tissue grafts.
Abstract
Regeneration of oral soft tissue defects, including mucogingival defects associated with the recession or loss of gingival and/or mucosal tissues around teeth and implants, is crucial for restoring oral tissue form, function, and health. This study presents a novel approach using three-dimensional (3D) bioprinting to fabricate individualized grafts with precise size, shape, and layer-by-layer cellular organization. A multicomponent polysaccharide/fibrinogen-based bioink is developed, and bioprinting parameters are optimized to create shape-controlled oral soft tissue (gingival) constructs. Rheological, printability, and shape-fidelity assays, demonstrated the influence of thickener concentration and print parameters on print resolution and shape fidelity. Artificial intelligence (AI)-derived tool enabled streamline the iterative bioprinting parameter optimization and analysis of the interaction between the bioprinting parameters. The cell-laden polysaccharide/fibrinogen-based bioinks exhibited excellent cellular viability and shape fidelity of shape-controlled, full-thickness gingival tissue constructs over the 18-day culture period. While variations in thickener concentrations within the bioink minimally impact the cellular organization and morphogenesis (gingival epithelial, connective tissue, and basement membrane markers), they influence the shape fidelity of the bioprinted constructs. This study represents a significant step toward the biofabrication of personalized soft tissue grafts, offering potential applications in the repair and regeneration of mucogingival defects associated with periodontal disease and dental implants.