STAT+: What Baby KJ means for the CRISPR gene editing industry
The custom-built gene editing treatment for 6-month-old KJ Muldoon could not have come at a more welcome or jarring time for CRISPR-powered biomedicine.

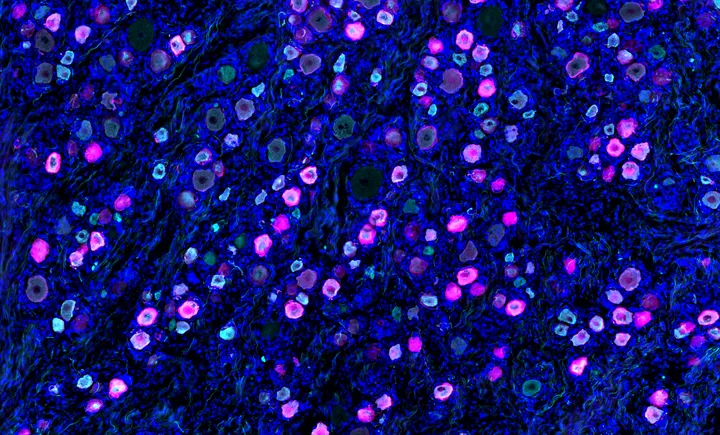
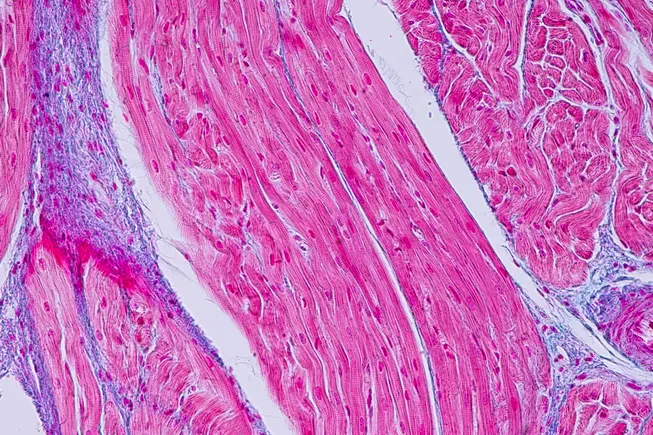
For the ailing gene editing industry, hope came earlier this month in the tiny, smiling, fuzzy-headed form of KJ Muldoon.
At just 6 months old, KJ received a gene editing treatment custom-built to correct his unique mutation. He’s not cured, researchers explained at the annual American Society of Gene & Cell Therapy meeting in New Orleans. But he has been able to resume a normal diet and is no longer on the path to a liver transplant.
The news could not have come at a more welcome or more jarring time for the field. For three years, gene editing has seemed in free fall, riven by layoffs, closures, shuttered programs and sinking stock prices. Now here, finally, in striped pajama form, was a reminder of what a decade of advances could deliver. “How awesome is it that we’re at this point?” said Nessan Bermingham, who co-founded and led Intellia, one of the first CRISPR companies, until 2017.



























































































![[Video] The Weekly Break Out Ep. 19: Army aviation’s shakeup and the F-35’s future](https://breakingdefense.com/wp-content/uploads/sites/3/2025/05/EP-19-THUMB-play-button.jpg?#)