Self-sacrifice of sulfide electrolytes facilitating stable solid-state sodium–sulfur batteries
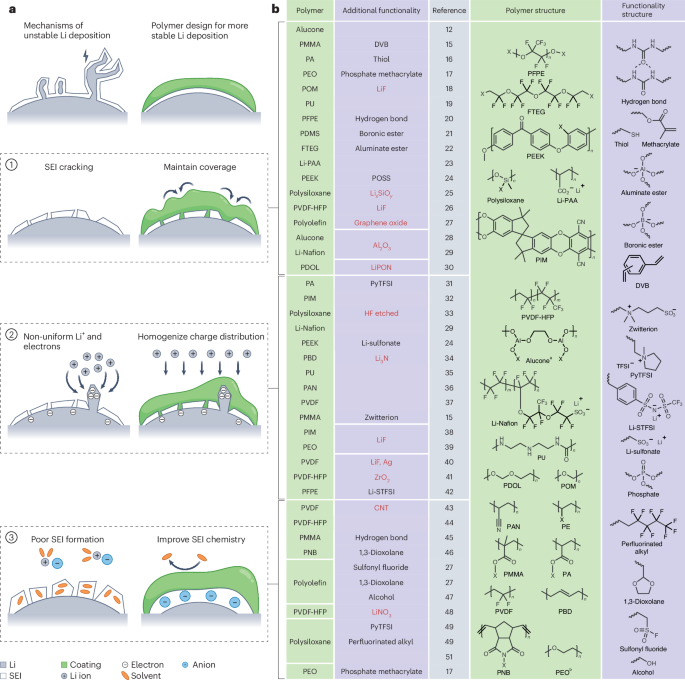
Energy Environ. Sci., 2025, 18,4288-4301DOI: 10.1039/D4EE06171C, Paper Open Access   This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.Yi Yuan, Yang Hu, Yi Gan, Zhiliang Dong, Yijia Wang, Enzhong Jin, Mingrui Yang, Frederick Benjamin Holness, Vinicius Martins, Qingsong Tu, Yang ZhaoA sulfide solid electrolyte, Na3SbS4, undergoes a simultaneous self-redox process during the operation of solid-state Na–S batteries. The in situ construction of interfaces aids in overcoming the intrinsic issues on both the cathode and anode sides.The content of this RSS Feed (c) The Royal Society of Chemistry

DOI: 10.1039/D4EE06171C, Paper
 Open Access
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.A sulfide solid electrolyte, Na3SbS4, undergoes a simultaneous self-redox process during the operation of solid-state Na–S batteries. The in situ construction of interfaces aids in overcoming the intrinsic issues on both the cathode and anode sides.
The content of this RSS Feed (c) The Royal Society of Chemistry





















































































![The F-35’s future: The power and cooling competition that could change everything [Video]](https://breakingdefense.com/wp-content/uploads/sites/3/2024/09/240924_F35_moon_USAF-scaled-e1727200160419.jpg?#)