Optimization of Adsorption Sites for Selective Hydrobenzoin and Syngas Production in a Single Photoredox Cycle
Advanced Energy Materials, EarlyView.

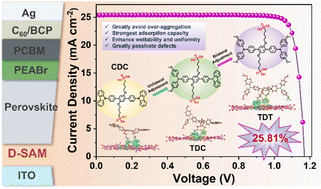
Efficient C─C coupling hydrobenzoin production and tunable syngas generation via benzyl alcohol oxidation and CO2 reduction is realized by Ni2P/CdS composite in a single photoredox cycle. In situ and ex situ characterizations, along with theoretical calculations, reveal that the active site optimization facilitates intermediate formation and desorption. Meanwhile, adjusting Ni2P content in Ni2P/CdS promotes H2 evolution, controlling the CO/H2 ratio of syngas.
Abstract
Integrating benzyl alcohol oxidation with carbon dioxide (CO2) reduction in a single photoredox catalysis is of high economic and practical interest. However, it remains challenging to controllably regulate the selectivity of specific C─C coupling chemicals (oxidation products) and the ratio of carbon monoxide and hydrogen (CO/H2) for syngas (reduction products). Herein, an efficient photocatalyst consisting of CdS nanorods decorated by Ni2P (NP/CdS) is developed, which achieves remarkable performance, producing C─C coupling hydrobenzoin (HB) with an excellent yield of ≈315.4 µmol g−1 h−1 and selectivity of ≈90%. This performance originates from the optimized adsorption of benzaldehydes and protons, promoting the generation of the critical radical intermediates (•CH(OH)Ph). Meanwhile, the favorable desorption of •CH(OH)Ph and HB from the binding sites is attained. On the other hand, by increasing the Ni2P content in NP/CdS, the CO/H2 ratio can be adjusted across a wide range (from ≈15:1 to ≈2.6:1), enabling syngas compositions suitable for industrial feedstock applications. This tunability is attributed to the lower CO2 affinity of the Ni2P phase compared to CdS while demonstrating higher activity for H2 evolution. This work presents a novel approach for selectively and efficiently producing HB and tunable syngas simultaneously.