NIR‐II‐Responsive Chainmail Nanocatalysts for Spatiotemporally Controlled Enzymatic Tumor Therapy
Advanced Healthcare Materials, EarlyView.

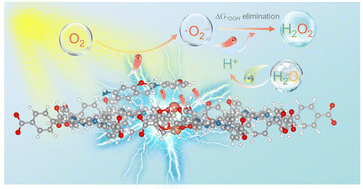
This work introduces a chainmail nanocatalyst, featuring nitrogen-doped carbon-encapsulated nanoceria, that remains inertness under physiological conditions but activates precise enzymatic functions under photoirradiation. It generates targeted oxidative damage in tumors by amplifying hydroxyl radicals while blocking antioxidant defenses. The design minimizes side effects and enhances therapeutic precision, offering a safer and more effective strategy for cancer treatment.
Abstract
The clinical translation of metal-based peroxidase-like nanozymes for antitumor therapy faces two critical challenges: off-target catalytic activation and suboptimal hydroxyl radical (•OH) generation efficiency. To address these limitations, an innovative chainmail nanocatalyst featuring nitrogen-doped carbon-encapsulated nanoceria is developed, which combines spatial confinement effects with photo-trigger catalytic enhancement. The graphitic carbon shell serves as a physical barrier that effectively isolates metallic cerium from the biological environment, reducing nonspecific catalytic activation by 100% compared to bare nanoceria. Remarkably, under 1064 nm laser irradiation, electrons of cerium species can penetrate the carbon confinement through quantum tunneling effects, activating multiple enzymatic pathways. Vacancy engineering further optimizes the Ce3+/Ce4+ redox pair ratio (1.75 vs 0.44 in pristine nanoceria), establishing an electron reservoir that facilitates catalytic amplification of H2O2-to-•OH conversion and glutathione oxidase-mimicking activity for tumor microenvironment remodeling. This dual mechanism synergistically elevates intracellular oxidative stress while preserving normal tissue viability. In vivo evaluations demonstrate that the photoactivated nanocatalyst exhibits remarkable tumor suppression efficacy, prolonging the survival duration of tumor-bearing mice from 33 days to 70 days. The light-gated chainmail architecture provides a paradigm for spatiotemporally controlled catalytic therapy, resolving the critical dilemma between catalytic potency and biological specificity in nanozyme design.