GlycoEra’s global jobs underscore U.S. brain drain
And more biotech news brought to you by The Readout.

Hello! Hope you had a nice extended weekend. Today, we talk about gene editing, particularly as it relates to pushing the field forward and how to maintain human dignity in the process. Also, a setback for Prothena, and a case for why biohacking doesn’t make sense.
The need-to-know this morning
- Rocket Pharmaceuticals said a child with Danon disease, a severe, inherited heart condition, died after receiving treatment with the company’s gene therapy in a clinical trial. The cause of death is still being investigated but Rocket said the patient experienced complications due to capillary leak syndrome, possibly tied to an immune-suppressing treatment used prior to the patient receiving the Rocket gene therapy, called RP-A501. The FDA placed the study on clinical hold.
- Biogen and City Therapeutics, a privately held developer of RNA-based “trigger molecules,” announced a partnership to develop a drug for an undisclosed central nervous system disease. The research is still in preclinical development. City was co-founded by veteran biotech executive John Maraganore.
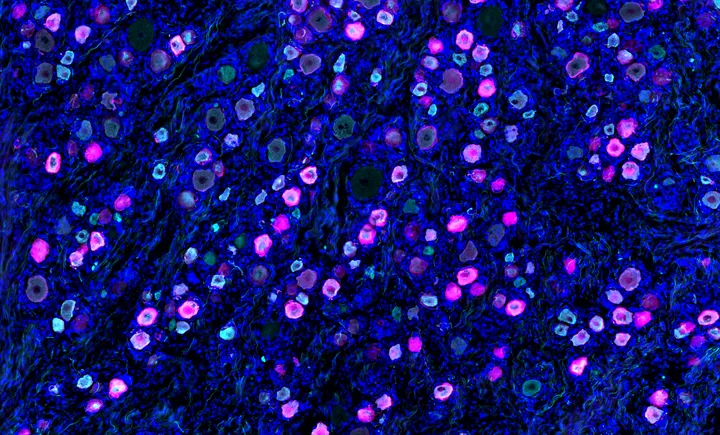
Baby’s custom-built CRISPR treatment revitalizes gene therapy industry
The success of a custom-built CRISPR treatment for baby KJ Muldoon — who has avoided a liver transplant thanks to a therapy that targets his unique mutation — has reenergized a struggling gene editing field, STAT’s Jason Mast writes. Yet while the case shows the lifesaving potential of n-of-1 therapies, it also highlights steep challenges: limited organ targets, unsustainable costs, murky regulatory pathways, and a lack of financial incentive.



























































































![[Video] The Weekly Break Out Ep. 19: Army aviation’s shakeup and the F-35’s future](https://breakingdefense.com/wp-content/uploads/sites/3/2025/05/EP-19-THUMB-play-button.jpg?#)