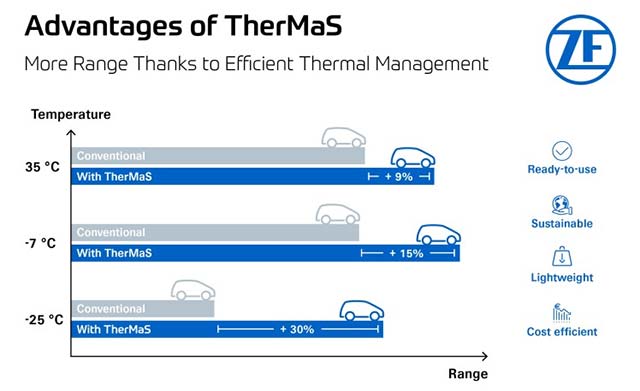
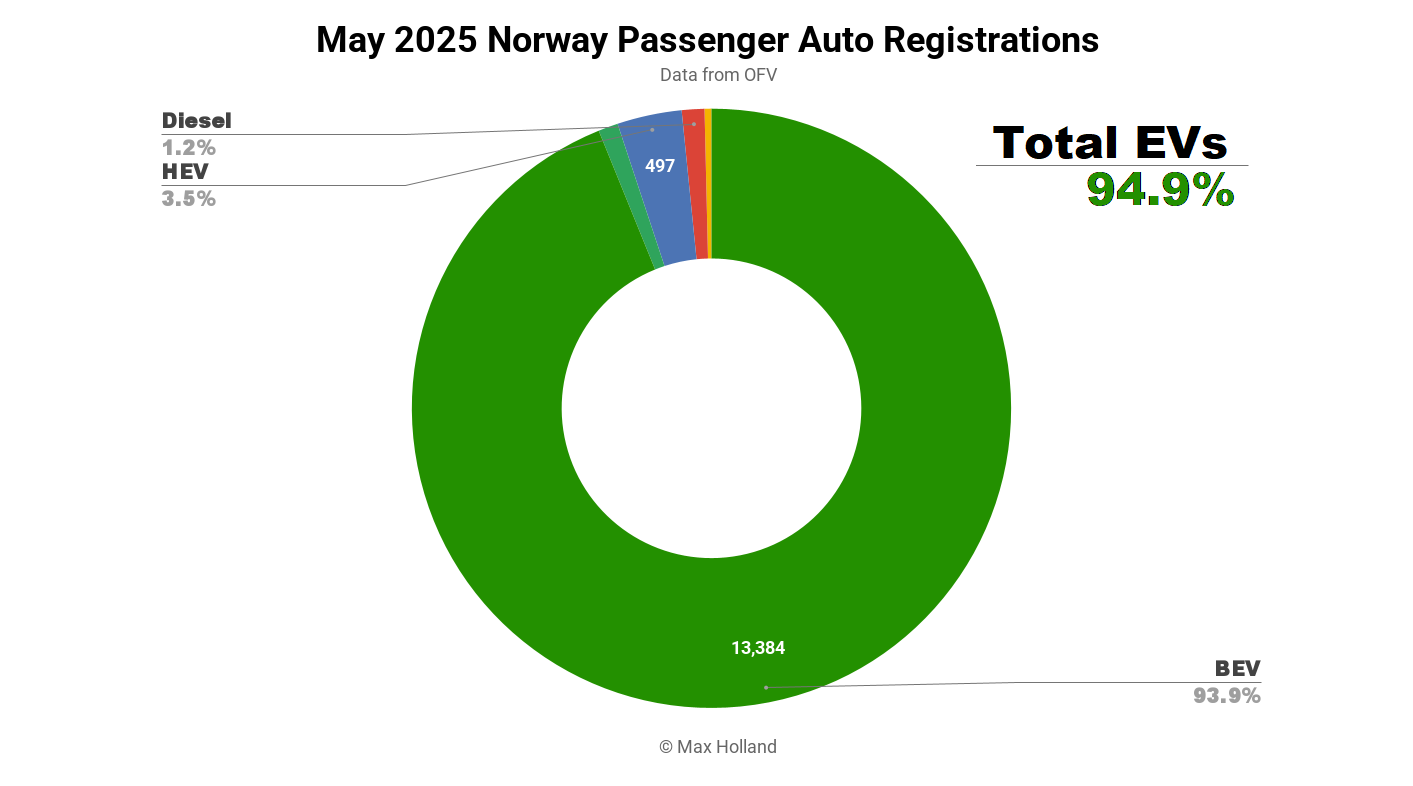
Penumbra announces FDA clearance and launch of detachable embolisation coil
Penumbra has introduced Ruby XL System, the detachable embolisation coil, which has also received clearance from the US Food and Drug Administration (FDA). The post Penumbra announces FDA clearance and launch of detachable embolisation coil appeared first on Medical Device Network.

The post Penumbra announces FDA clearance and launch of detachable embolisation coil appeared first on Medical Device Network.