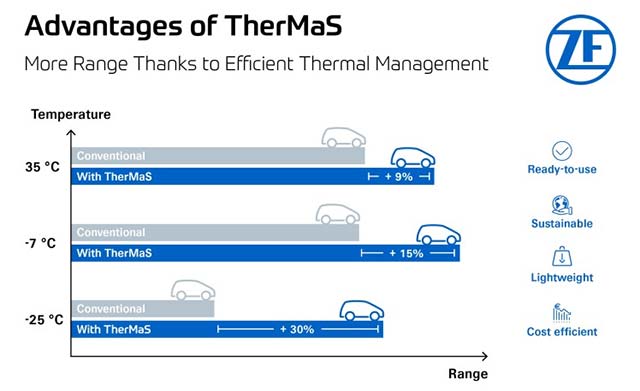
Isoflurane activates the type 1 ryanodine receptor to induce anesthesia in mice
by Hiroyuki J. Kanaya, Ken Kuwajima, Yuko Ito, Yuta Shinohara, Yohei Okubo, Shinnosuke Shiono, Fumiya Tatsuki, Rei-ichiro Ohno, Hideki Ukai, Maki Ukai-Tadenuma, Kenta Sumiyama, Hiroshi Fujishima, Rikuhiro G. Yamada, Daisuke Tone, Hiroshi Kiyonari, Masaki Kikuchi, Takashi Umehara, Takashi Murayama, Kazunori Kanemaru, Masamitsu Iino, Koji L. Ode, Takatsugu Hirokawa, Hiroki R. Ueda Inhaled anesthetics were first introduced into clinical use in the 1840s. Molecular and transgenic animal studies indicate that inhaled anesthetics act through several ion channels, including γ-aminobutyric acid type A receptors (GABAARs) and two-pore domain K+ (K2P) channels, but other targets may mediate anesthetic effects. Mutations in the type 1 ryanodine receptor (RyR1), which is a calcium release channel on the endoplasmic reticulum membrane, are relevant to malignant hyperthermia, a condition that can be induced by inhaled anesthetics. However, it was previously uncertain whether inhaled anesthetics directly interact with RyR1. In our study, we demonstrated that isoflurane and other inhaled anesthetics activate wild-type RyR1. By employing systematic mutagenesis, we discovered that altering just one amino acid residue negates the response to isoflurane, thus helping us to pinpoint the potential binding site. Knock-in mice engineered to express a mutant form of RyR1 that is insensitive to isoflurane exhibited resistance to the loss of righting reflex (LORR) when exposed to isoflurane anesthesia. This observation suggests a connection between RyR1 activation and the anesthetic effects in vivo. Moreover, it was shown that RyR1 is involved in the neuronal response to isoflurane. Additionally, administering new RyR1 agonists, which share the same binding site as isoflurane, resulted in a sedation-like state in mice. We propose that isoflurane directly activates RyR1, and this activation is pertinent to its anesthetic/sedative effects.
by Hiroyuki J. Kanaya, Ken Kuwajima, Yuko Ito, Yuta Shinohara, Yohei Okubo, Shinnosuke Shiono, Fumiya Tatsuki, Rei-ichiro Ohno, Hideki Ukai, Maki Ukai-Tadenuma, Kenta Sumiyama, Hiroshi Fujishima, Rikuhiro G. Yamada, Daisuke Tone, Hiroshi Kiyonari, Masaki Kikuchi, Takashi Umehara, Takashi Murayama, Kazunori Kanemaru, Masamitsu Iino, Koji L. Ode, Takatsugu Hirokawa, Hiroki R. Ueda Inhaled anesthetics were first introduced into clinical use in the 1840s. Molecular and transgenic animal studies indicate that inhaled anesthetics act through several ion channels, including γ-aminobutyric acid type A receptors (GABAARs) and two-pore domain K+ (K2P) channels, but other targets may mediate anesthetic effects. Mutations in the type 1 ryanodine receptor (RyR1), which is a calcium release channel on the endoplasmic reticulum membrane, are relevant to malignant hyperthermia, a condition that can be induced by inhaled anesthetics. However, it was previously uncertain whether inhaled anesthetics directly interact with RyR1. In our study, we demonstrated that isoflurane and other inhaled anesthetics activate wild-type RyR1. By employing systematic mutagenesis, we discovered that altering just one amino acid residue negates the response to isoflurane, thus helping us to pinpoint the potential binding site. Knock-in mice engineered to express a mutant form of RyR1 that is insensitive to isoflurane exhibited resistance to the loss of righting reflex (LORR) when exposed to isoflurane anesthesia. This observation suggests a connection between RyR1 activation and the anesthetic effects in vivo. Moreover, it was shown that RyR1 is involved in the neuronal response to isoflurane. Additionally, administering new RyR1 agonists, which share the same binding site as isoflurane, resulted in a sedation-like state in mice. We propose that isoflurane directly activates RyR1, and this activation is pertinent to its anesthetic/sedative effects.