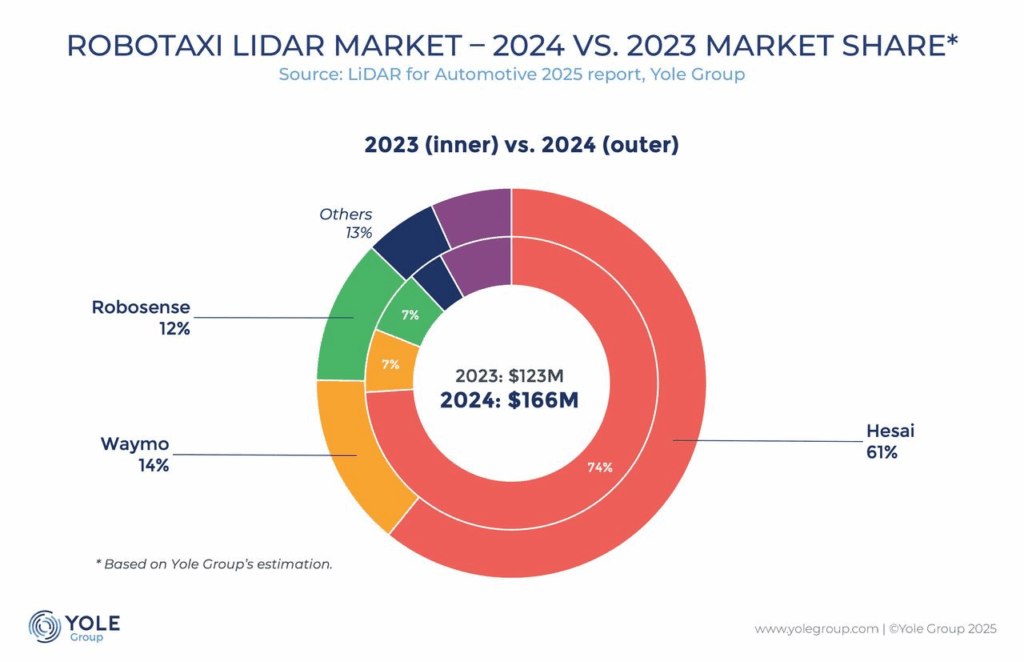
STAT+: Pharmalittle: We’re reading about a Trump drug-pricing plan, a new FDA official trying to rally staff, and more
President Trump plans to revive an effort to tie what the government pays for some medicines to lower prices abroad

Top of the morning to you, and a fine one it is. Crystal clear blue skies and pleasant breezes are enveloping the Pharmalot campus, where the official mascots are attempting to intimidate various creatures and the commuter traffic is whizzing by. As for us, we are busy brewing another cup of stimulation. Our choice today is hazelnut creme, an old standby. Please feel free to join us. Of course, we are also prowling for items of interest and are passing along the list below to help you get started on your own journey today. With that said, we hope all goes well and that you conquer the world. Best of luck, and do keep in touch. …
President Trump plans to revive an effort to dramatically slash drug costs by tying the amount the government pays for some medicines to lower prices abroad, according to Politico. Trump early next week is expected to sign an executive order directing aides to pursue the initiative, called “most favored nation,” for a selection of drugs within the Medicare program. The idea would use the administration’s authorities to force prices down. The proposal has not been finalized and could still change as aides work through the specifics, said the people involved in the plan, who were granted anonymity to describe internal deliberations. Trump has not yet personally approved the plan. The president on Tuesday teased a “very big announcement” within the next week that one of the people familiar characterized as a reference to the drug-pricing proposal.
In his first address to U.S. Food and Drug Administration staff on Wednesday, Vinay Prasad emphasized his commitment to evidence, his admiration for agency employees, and his desire to serve the American public, STAT tells us. The new director of the Center for Biologics Evaluation and Research sounded very different from his online firebrand persona. He struck a humble, self-deprecating, and even earnest tone, calling the American people the agency’s “number one stakeholder.” “My academic research disciplines epidemiology, evidence-based medicine,” Prasad said in a recording obtained by STAT. “I applaud what you do. I’ve learned a lot from reading your papers over the last 20 years.” Prasad, a hematologist and oncologist, will oversee the nation’s vaccines, gene therapies, and the blood supply as CBER director. He has sharply criticized the FDA in the past, accusing regulators of being too close with the pharmaceutical industry and falling short on safety and efficacy standards. Last year, he said Americans would be better off without the FDA at all than with the agency in its current form.