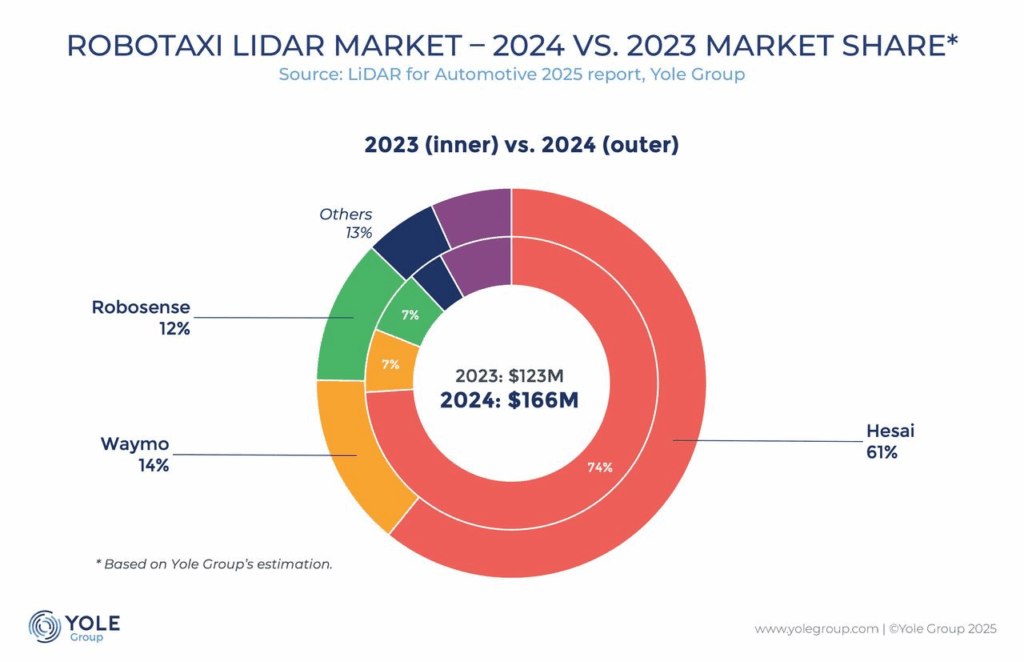
FDA misses another approval decision target date, this time for GSK's Nucala in COPD
GSK had expected to secure an FDA nod for its IL-5 antibody Nucala in chronic obstructive pulmonary disease on Wednesday, but the May 7 deadline has come and gone without a decision from the U.S. regulator. The situation marks the latest in a string of missed deadlines by the FDA following mass job cuts under the Trump administration.