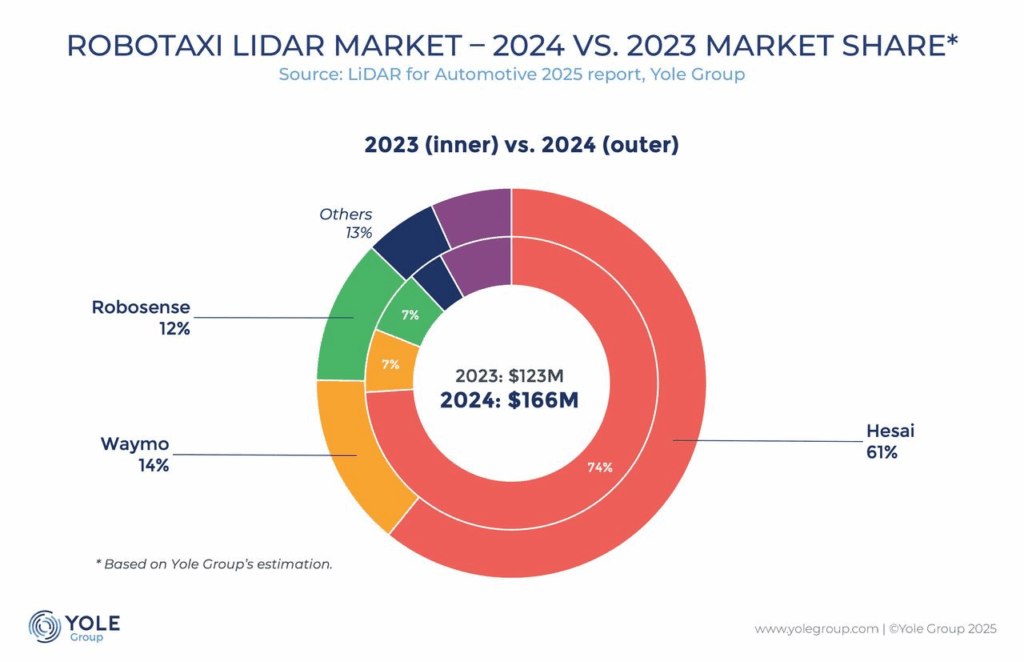
Ion Redistribution Gel Electrolyte Dissipates Interfacial Turbulence for Aqueous Zinc‐Ion Batteries
Advanced Energy Materials, Volume 15, Issue 15, April 15, 2025.

Enhanced concentration polarization and charge accumulation can lead to failure in aqueous zinc-ion batteries (AZBs). An ion redistribution gel electrolyte with functional carboxymethyl groups (PCMS) is used to preferentially regulate the ion concentration at the electrode-electrolyte interface. This PCMS gel electrolyte achieves excellent electrochemical stability at high current densities, advancing the development of highly reversible AZBs.
Abstract
Aqueous zinc-ion batteries (AZBs) degrade under a high current density due to the existence of interfacial turbulence with severe concentration polarization. Herein, an ion redistribution gel electrolyte is introduced to stabilize the electrode-electrolyte interface by regulating the ion density gradient. Negatively charged carboxymethyl groups in carboxymethyl starch ether polymer (PCMS) electrolytes can preferentially regulate the ion concentration at the anode interface. The quasi-solid PCMS gel electrolytes can dissipate the charge accumulation caused by ion concentration differences, which is beneficial for achieving excellent electrochemical stability at high current densities. As a result, the symmetric Zn cells with PCMS gel electrolyte can be cycled for more than 2000 h at a high current density of 40 mA cm−2. Furthermore, the Zn//I2 cell using PCMS gel electrolyte also sustained cycling for over 8000 cycles, and the pouch cell with PCMS gel electrolyte exhibited practical deformable applications in flexible devices. This work highlights an effective gel electrolyte approach for enhancing interfacial stability, potentially advancing the development of highly reversible AZBs.