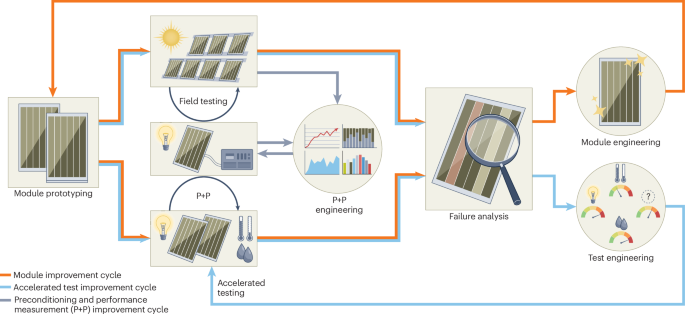
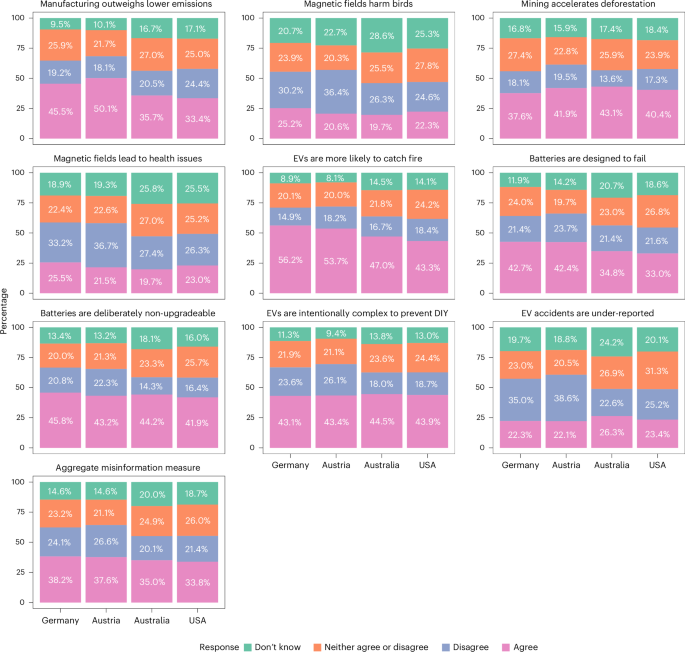
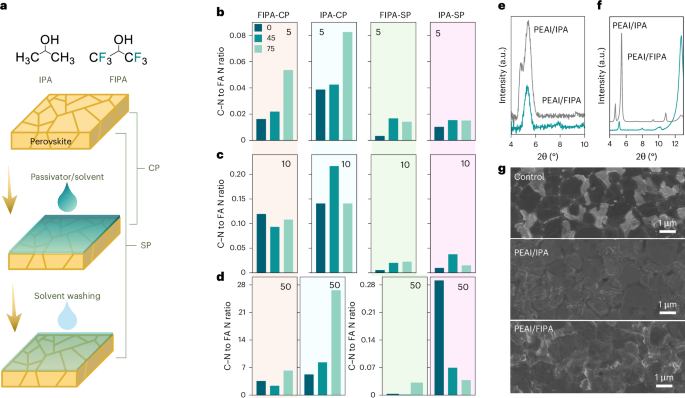
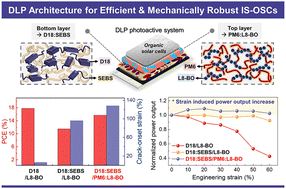
FDA clears Neurent Medical’s NEUROMARK for chronic rhinitis treatment
The US FDA has granted 510(k) clearance to Neurent Medical's NEUROMARK System for the treatment of individuals with chronic rhinitis. The post FDA clears Neurent Medical’s NEUROMARK for chronic rhinitis treatment appeared first on Medical Device Network.

The post FDA clears Neurent Medical’s NEUROMARK for chronic rhinitis treatment appeared first on Medical Device Network.