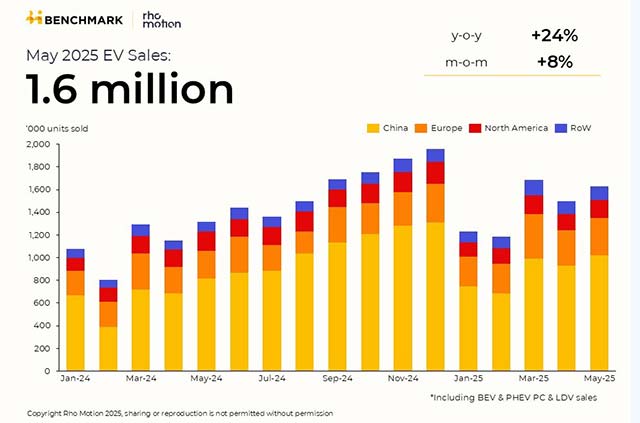
EHA: GSK adds fuel to Blenrep's comeback case ahead of potential US return
GSK's multiple myeloma antibody drug conjugate Blenrep is up for an FDA decision in July. If approved, the drug would complete a return to the U.S. market after it was withdrawn following a failed confirmatory study in 2022.